CARTISTEM
1) CARTISTEM, the Allogeneic Umbilical Cord Blood-derived Mesenchymal Stem Cell drug developed by Medipost; One of the ten stem cell drugs in the world.
2) It has been approved of its commercial use by the Ministry of Food & Drug Safety in January 2012. Become the first stem cell drug for the treatment of knee osteoarthritis, breaking the traditional concept of cartilage cannot be regenerate.
3) Dosage Form: a suspension of hUCB-MSC in hyaluronic acid (gel matrix)

Stem cells are undifferentiated biological cells that can differentiate into specialized cells and can divide (through mitosis) to produce more stem cells. They are found in multicellular organisms. In mammals, there are two broad types of stem cells: embryonic stem cells, which are isolated from the inner cell mass of blastocysts, and adult stem cells, which are found in various tissues. In adult organisms, stem cells and progenitor cells act as a repair system for the body, replenishing adult tissues.
Indication:Repair of knee cartilage defects in patients with osteoarthritis (ICRS grade IV) as a result of degeneration or repetitive trauma.
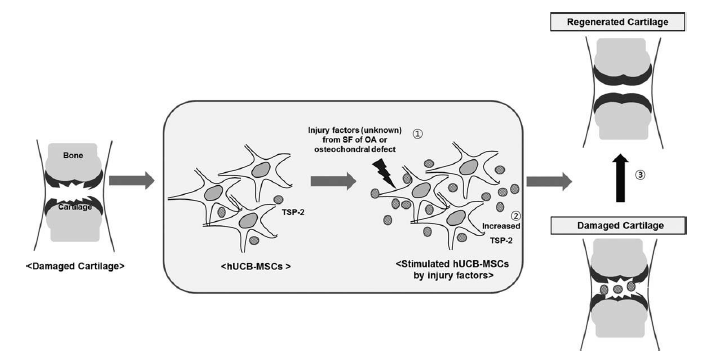
hUCB-MSCs transplanted into damaged cartilage were stimulated by unknown injury factors in the SF from OA patients to increased TSP-2 secretion. This TSP-2 diffused into synovial cavity and induced differentiation of chondroprogenitor cells. By means of this paracrine action of at least TSP-2, hUCB-MSCs accomplishes regeneration of damaged cartilage.
Advantages:
1. Sole stem cell drug has been approved of its commercial use in arthritis in the world
2. Not subject to age restriction, only one operation can be cured
3. Sustained and excellent support for the regenerate cartilage within 7 years
4.The results of the Phase III clinical trial showed a effective rate of 97.7%
5. Small surgical trauma, Remarkable curative effect for large area of cartilage damage
6. Simple operation procedure and short hospitalization time
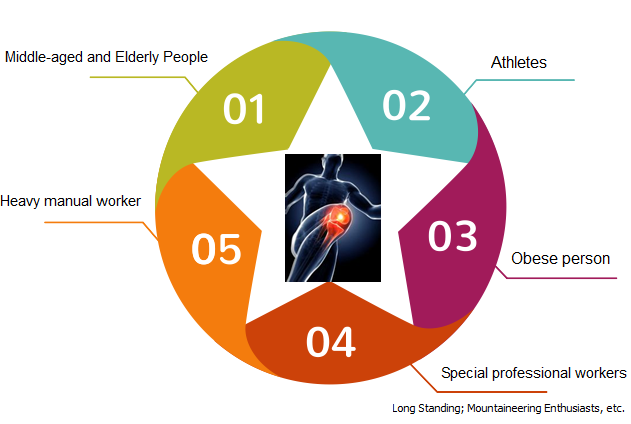
The target patient of CARTISTEM is the person with ICRS IV OA caused by repeated trauma or degenerative. CARTISTEM is the first treatment option for severe OA patients in the age group between 50s and 60s at the moment. On the other hand, the target patient without limitation by age. By far, the youngest patient was at the age of 12 and the oldest was at the age of 94.